Salt Water Systems With High Calcium Hardness
Posted by Ken Scheer on Dec 19, 2016
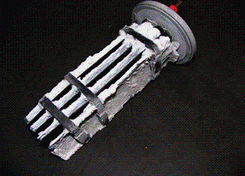
Salt systems have been extremely popular for many years now and when they are functioning properly it can make your swimming experience in your pool quite enjoyable. For most, the water can feel "softer"; it's easier to manage chemistry (chlorine levels) and leaves you feeling much cleaner when you get out of the swimming pool! But, what happens when the calcium hardness levels rise in the swimming pool causing the salt system to not function correctly? This is a common problem, especially in the United States where calcium and other hardness mineral levels are extremely high in our fill water! When individuals and commercial properties purchase salt systems, this isn’t something that is usually brought up and unfortunately when calcium attaches itself to the salt cell, it no longer works. Sure…. you can acid bath the cell but that takes life off of your cell, you could drain it and refill it with hard water again.
The biggest challenge one will always have with salt systems is as water flows through the cell and along the electrode plates it begins to scale because the water is so hard. When the scale becomes thick the electrode plates will no longer manufacture chlorine. As the salt water flows through the cell, a low-voltage direct current is applied to flat, rectangular plates inside the cell, initiating electrolysis. Through electrolysis, salt and water break up into hydrogen gas and hypochlorous acid. The hydrogen gas simply leaves the swimming pool water in the form of small bubbles. The hypochlorous acid sanitizes the swimming pool water and ultimately reverts back into salt, and the the process repeats. This process stops when calcium levels are too high, which means you will need to add chlorine to the pool or change out the water!
If you are looking to just replace your salt chlorine generator cell or chlorinator parts click here.
